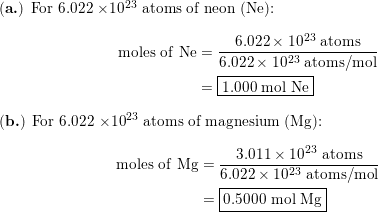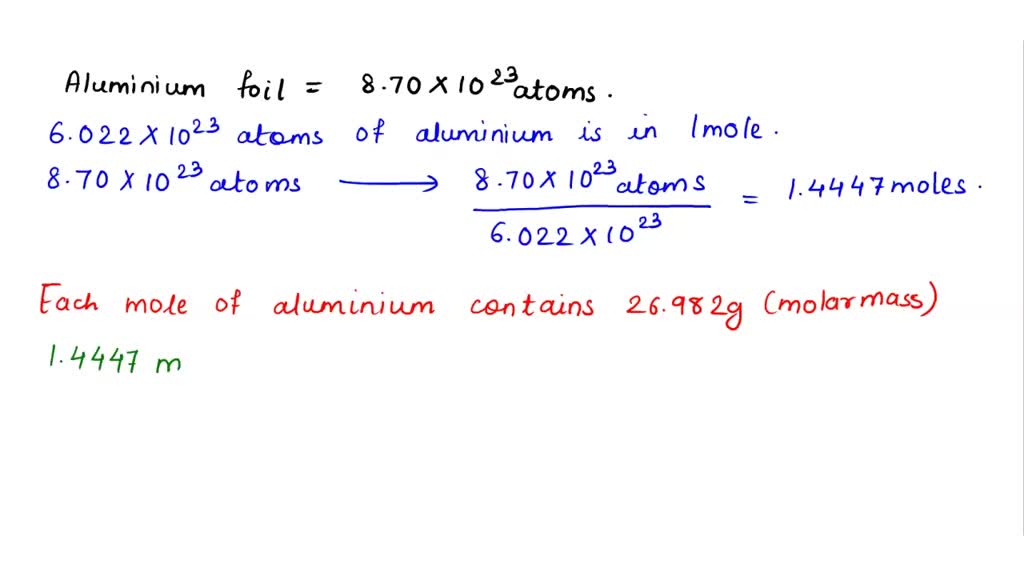
SOLVED: Question 13 of 13 A sample of aluminum foil contains 8.70 X 1023 atoms. What is the mass of the foil? mass of foil

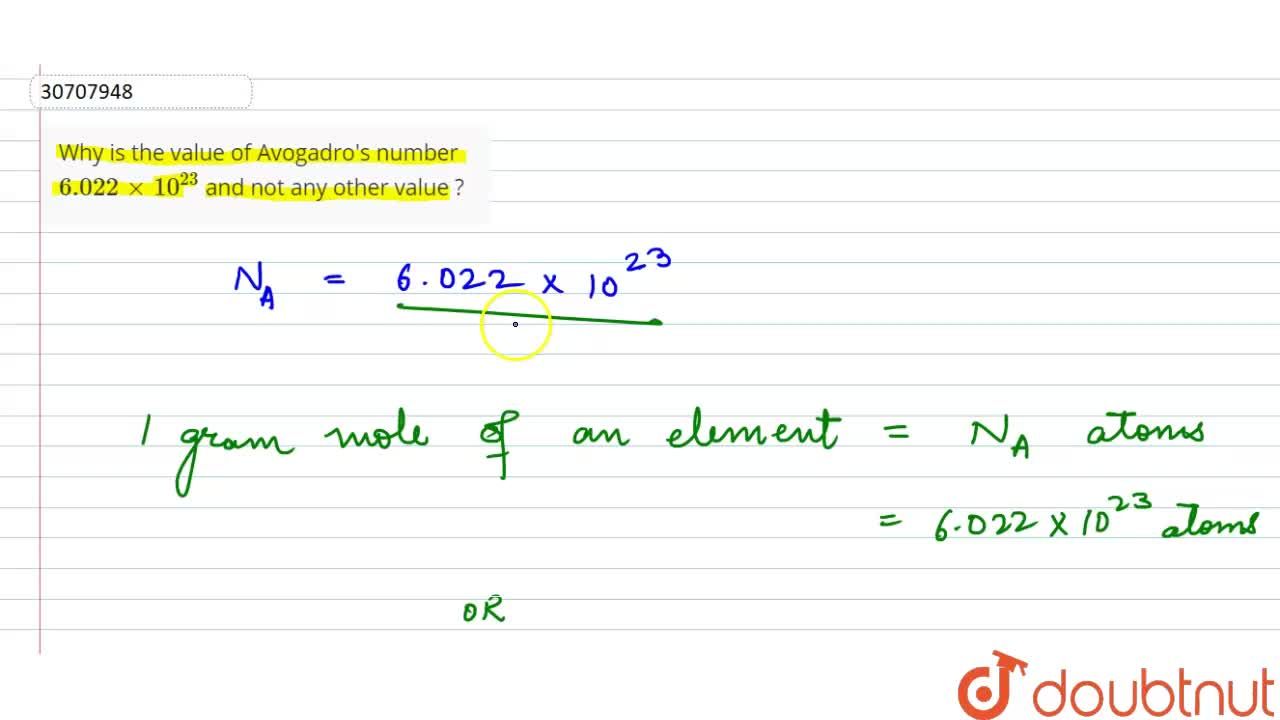
If Avogadro number NA is changed from 6.022 × 10^23 mol^-1 to 6.022 × 10^20 mol^-1 , this would change :

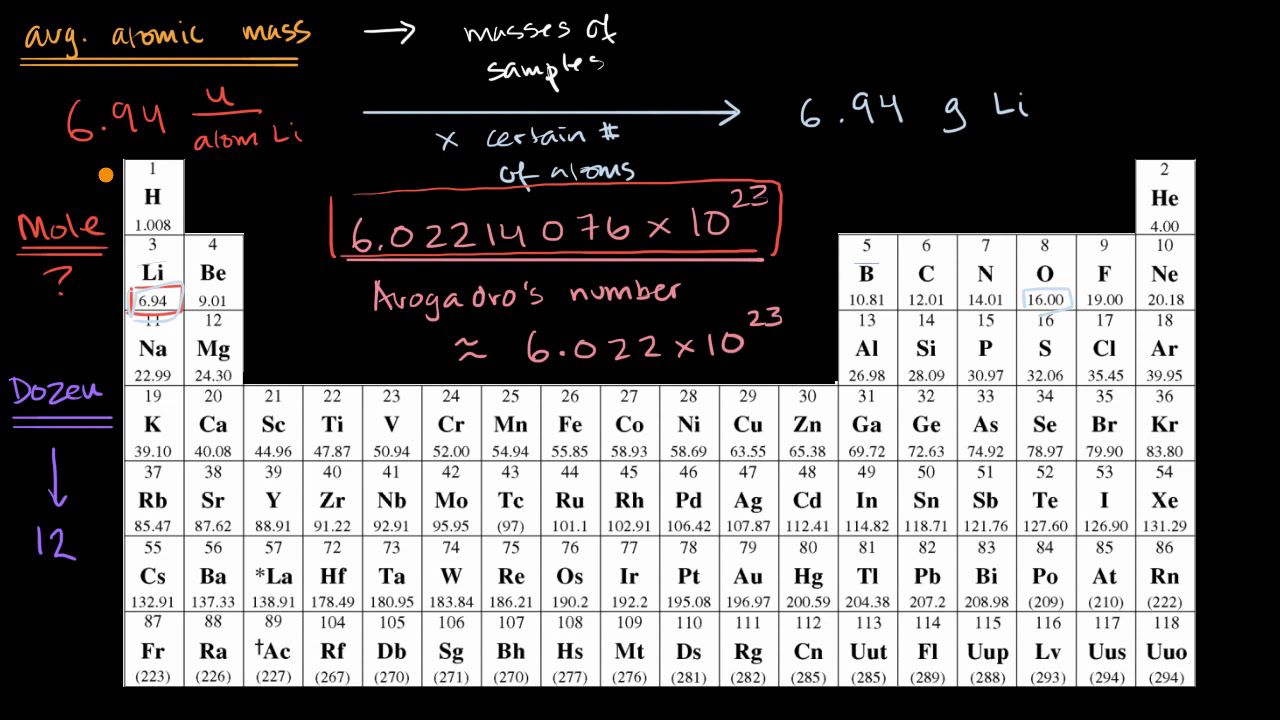
1 mole = 6 022 x 10^23 If there is 1 mole of H2 we have multiply the Avogadro no - Science - Atoms and Molecules - 15776529 | Meritnation.com

Which of the following contains the same number of atoms as 4.032g of hydrogen atoms? A. 1 mole of H2 - Brainly.com
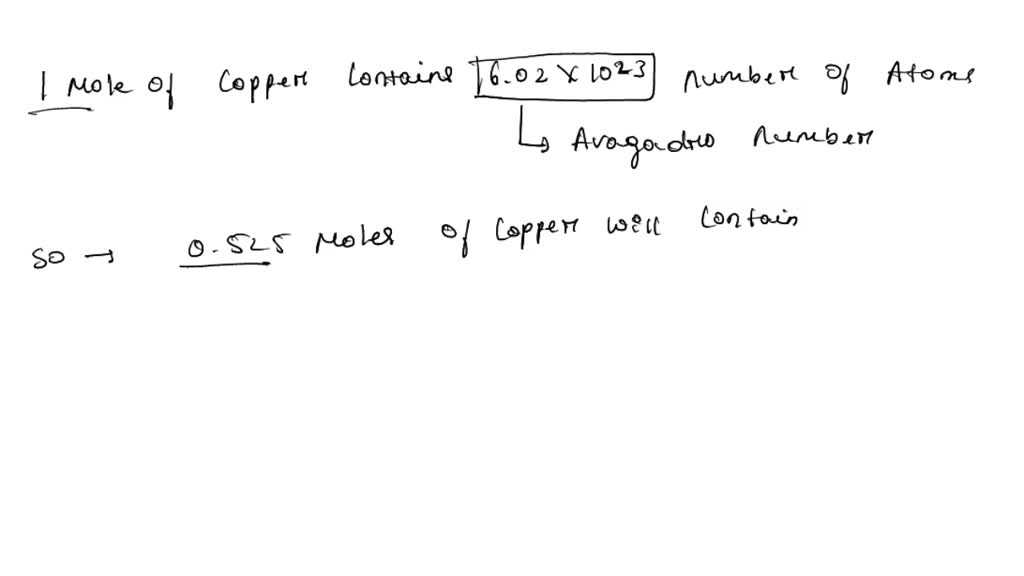
SOLVED: Knowing that Avogadro number is equal to 6.022 x 1023 atoms/mol, do the following: How many moles of iron are in 50.0 g of iron? How many moles of copper atoms

SOLVED: 2. One mole of water molecules contains: a) 18.02 g of water b) 6.022 x 1023 atoms c) 6.022 x 1023 molecules of water d) both a) and c) 3 How

Calculate the mass of 6 022 x 10^23 molecules of CaCO3 - Science - Atoms and Molecules - 13283691 | Meritnation.com