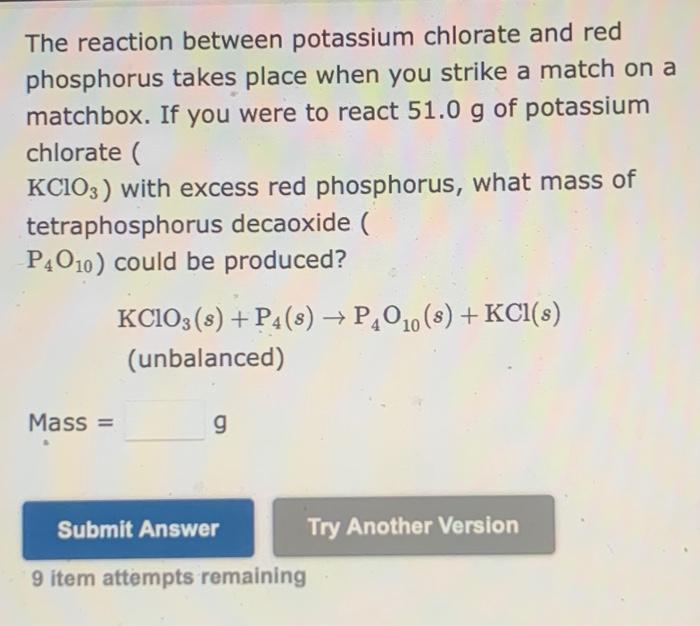
SOLVED:The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 52.9 g of potassium chlorate (KClO3) with excess red

SOLVED: The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 35.5 g of potassium chlorate (KClO3) with excess

jBK.pittiKUMAR. on Twitter: "The head of safetyMATCHES/ @matchBOX /vathiKUCHI r made of an oxidizing agent( #KclO3 #potassiumCHLORATE). The side of the box contains red #PHOSPHOROUS #P+powdered glass. https://t.co/vFHw94bZne https://t.co/9ivOkN7JRK ...

SOLVED:The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 52.9 g of potassium chlorate (KClO3) with excess red

SOLVED: 5. The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox: Ifyou were to react 34.5 g of potassium chlorate (KCIO3) with excess
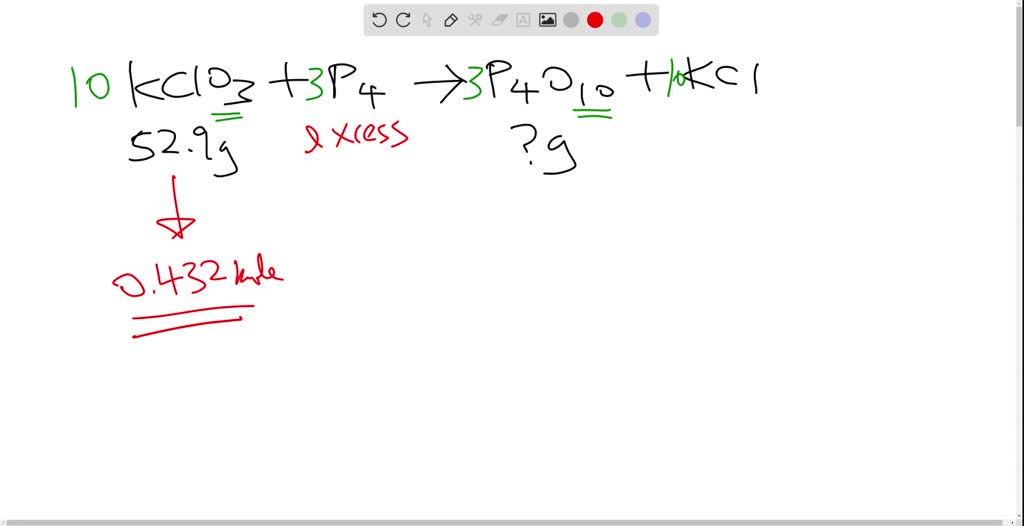
SOLVED:The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 52.9 g of potassium chlorate (KClO3) with excess red

SOLVED:The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 52.9 g of potassium chlorate (KClO3) with excess red